Step-by-step explanation:
An allotrope is defined as one or more form of a chemical element that exist in same physical state but different chemical properties.
For example, allotropes of carbon are graphite and diamond. And, both of them exist in a solid state with different chemical properties.
On the other hand, an isotope is two or more forms of an element that contains same number of protons but different number of neutrons.
For example,
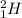 and
and
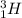 are isotopes of hydrogen.
are isotopes of hydrogen.
Some of the differences between allotropes and isotopes are as follows.
- Isotopes have different atomic masses but they show similar chemical properties due to the presence of same number of electrons.
- All allotropes are stable molecules that are found in nature. But in case of isotopes, some are stable while some are unstable.
- Almost all elements have isotopes. But all chemical elements does not have allotropes
one or more forms of a chemical element that occur in the same physical state. ... Allotropes may display very different chemical and physical properties. For example, graphite and diamond are both allotropes of carbon that occur in the solid state.