Step-by-step explanation:
A formula which represents proportions between the elements present in a compound is known as empirical formula.
Whereas a molecular formula is defined as the formula that represents total number of each element present in a compound.
When a molecular formula is able to be reduced to smaller whole-number ratio of the elements then both molecular and empirical formulas are considered to be the same.
Relation between molecular formula and empirical formula is as follows.
Molecular formula = n × Empirical formula
where, n is an integer.
For example, molecular formula of acetic acid is
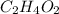 and molecular formula of formaldehyde is
and molecular formula of formaldehyde is
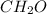 .
.
So, it means molecular formula of acetic acid can also be written as
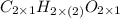 .
.
Therefore, we can conclude that both acetic acid and formaldehyde are two molecules that have different molecular formulas but the same empirical formula.