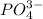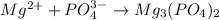Answer:The formulas of ionic compounds are:
a)
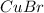
b)
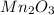
c)
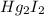
d)
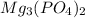
Step-by-step explanation:
Formulas for the an ionic compounds is determine by:
Criss-cross method, the oxidation state of the ions gets exchanged and they form the subscripts of the other ions. This results in the formation of a neutral compound.
(a) Copper bromide :Given that it contains
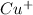 ion.
ion.
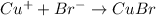
(b) Manganese oxide : Given that it contains
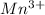 ion.
ion.
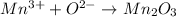
(c)Mercury iodide :Given that it contains
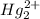
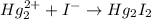
(d) Magnesium phosphate :Given that it contains