Answer:
(a)
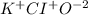 potassium hypochlorite.
potassium hypochlorite.
(b)
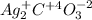 silver carbonate.
silver carbonate.
(c)
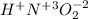 nitrous acid.
nitrous acid.
(d)
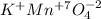 potassium permanganate.
potassium permanganate.
(e)
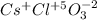 cesium chlorate.
cesium chlorate.
(f)
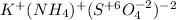 potassium ammonium sulfate.
potassium ammonium sulfate.
(g)
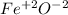 ferrous oxide.
ferrous oxide.
(h)
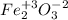 ferric oxide.
ferric oxide.
(i)
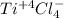 titanium chloride.
titanium chloride.
(j)
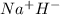 sodium hydride
sodium hydride
(k)
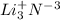 lithium nitride
lithium nitride
(l)
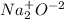 sodium oxide.
sodium oxide.
(m)
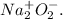 sodium peroxide (superoxide).
sodium peroxide (superoxide).
Step-by-step explanation:
Hello,
By following the IUPAC rule and each element's oxidation states, the names turn out into:
(a)
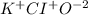 potassium hypochlorite.
potassium hypochlorite.
(b)
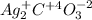 silver carbonate.
silver carbonate.
(c)
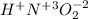 nitrous acid.
nitrous acid.
(d)
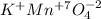 potassium permanganate.
potassium permanganate.
(e)
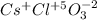 cesium chlorate.
cesium chlorate.
(f)
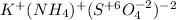 potassium ammonium sulfate.
potassium ammonium sulfate.
(g)
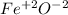 ferrous oxide.
ferrous oxide.
(h)
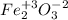 ferric oxide.
ferric oxide.
(i)
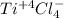 titanium chloride.
titanium chloride.
(j)
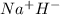 sodium hydride
sodium hydride
(k)
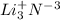 lithium nitride
lithium nitride
(l)
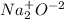 sodium oxide.
sodium oxide.
(m)
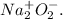 sodium peroxide (superoxide).
sodium peroxide (superoxide).
Best regards.