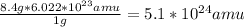Answer:
The number of amu in 8.4 g = 5.1*10^24 amu
Step-by-step explanation:
Given:
Mass in grams = 8.4 g
To determine:
The equivalent in amu (atomic mass units)
Calculation:
Unit conversion-
1 g = 6.022*10^23 amu
therefore the given mass is equivalent to: