Answer:
c. remain constant.
Step-by-step explanation:
The half life is defined as the time at which the reactant's concentration reduced to half.
The formula for the half life for a first order kinetic reaction is:
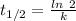
Where,
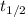 is the half life
is the half life
k is the rate constant.
As seen from the formula the half life for a first order reaction is independent of the reactant's concentration.
So, it will remain constant on any change of the reactant's concentration.