Answer: When oppositely charged species interact.
Step-by-step explanation:
Ionic compound is defined as the compound which is formed by the complete transfer of electrons from one element to another element.
In these compounds, oppositely charged ions are attracted towards each other to form a compound.
For Example: NaCl is formed by the attraction of
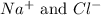 ions. So, same number of oppositely charges maintain electrical neutrality.
ions. So, same number of oppositely charges maintain electrical neutrality.
Electrical neutrality in a compound is achieved when atoms forming a compound have same number of opposite charges. Basically the charges on cation is balanced by the charges on anion.