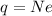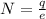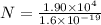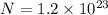Answer:
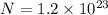
Step-by-step explanation:
As we know that the current through the battery is given as
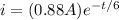
here from above equation we know that current will become zero when time elapsed is very large
so here we can say that charge will flow through the battery from t = 0 to t = infinite
now we have
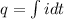
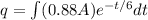
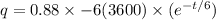
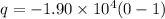
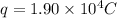
As we know that