Answer:
(a)
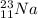
(b)
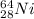
(c)
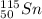
(d)
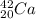
Step-by-step explanation:
Hello,
In this case, Z stands for the isotope's atomic number and A for the isotope's atomic mass, in such a way, looking for each isotope one concludes that the proper symbol, in the form of
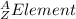 turn out being:
turn out being:
(a)
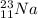
(b)
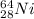
(c)
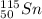
(d)
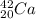
Best regards.