Step-by-step explanation:
A point of temperature at which both solid and liquid state of a substance remains in equilibrium without any change in temperature then this temperature is known as melting point.
For example, melting point of water is
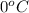 . So, at this temperature solid state of water and liquid state are present in equilibrium with each other.
. So, at this temperature solid state of water and liquid state are present in equilibrium with each other.
Therefore, when a 100 g of given pure metal in solid state is heated at its exact melting point which is
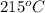 then some of the solid will change into liquid state but the temperature will remains the same.
then some of the solid will change into liquid state but the temperature will remains the same.