Answer:
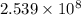 helium atoms will be needed to cover 1 inch distance.
helium atoms will be needed to cover 1 inch distance.
Step-by-step explanation:
Diameter of helium atom =
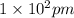
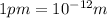
1 m = 39.3701 inch
Then in
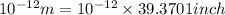
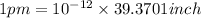
Diameter of helium atom in inches =

Let the number of helium atoms required to cover 1 inch distance be x.
Total distance = 1 inch
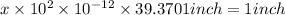
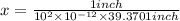
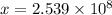 helium atoms
helium atoms
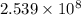 helium atoms will be needed to cover 1 inch distance.
helium atoms will be needed to cover 1 inch distance.