Answer: The atomic number of helium atom is 2 and mass number of helium atom is 4.
Step-by-step explanation:
Atomic number is defined as the number of protons or electrons that are present in a neutral atom. It is represented by the symbol 'Z'
Atomic number = number of protons = number of electrons
Mass number is defined as the sum of number of protons and neutrons that are present in an atom. It is represented by the symbol 'A'
Mass number = Number of protons + Number of neutrons
The general representation of an isotope is given as
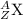
where,
A = mass number of element
Z = atomic number of element
X = symbol of the element
We are given:
An isotope of helium having formula of
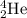
where,
Atomic number of helium = 2 = Number of protons = Number of electrons
Mass number of helium = Number of protons + Number of neutrons
4 = 2 + Number of neutrons
Number of neutrons = 4 - 2 = 2
Hence, the atomic number of helium atom is 2 and mass number of helium atom is 4.