Answer:
The pressure the hydrogen gas exerted before its volume was decreased is 2.7207 atm.
Step-by-step explanation:
Using Boyle's law
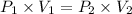
Given ,
V₁ = 14.65 L
V₂ = 7.94 L
P₁ = ?
P₂ = 5.02 atm
Using above equation as:
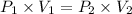
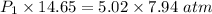
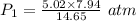
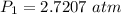
The pressure the hydrogen gas exerted before its volume was decreased is 2.7207 atm.