Answer:
a) volume of ammonium iodide required =349 mL
b) the moles of lead iodide formed = 0.0436 mol
Step-by-step explanation:
The reaction is:

It shows that one mole of lead nitrate will react with two moles of ammonium iodide to give one mole of lead iodide.
Let us calculate the moles of lead nitrate taken in the solution.
Moles=molarityX volume (L)
Moles of lead nitrate = 0.360 X 0.121 =0.0436 mol
the moles of ammonium iodide required = 2 X0.0436 = 0.0872 mol
The volume of ammonium iodide required will be:
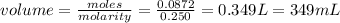
the moles of lead iodide formed = moles of lead nitrate taken = 0.0436 mol