Answer: The name of
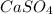 is calcium sulfate.
is calcium sulfate.
Step-by-step explanation:
An ionic compound is defined as the compound which is formed when electron gets transferred from one atom to another atom. These are usually formed when a metal reacts with a non-metal or a metal reacts with a polyatomic ion or a reaction between two polyatomic ions takes place.
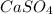 is an ionic compound because calcium element is a metal and sulfate ion is a polyatomic ion. The bond formed between a metal and a non-metal is always ionic in nature.
is an ionic compound because calcium element is a metal and sulfate ion is a polyatomic ion. The bond formed between a metal and a non-metal is always ionic in nature.
The nomenclature of ionic compounds is given by:
- Positive ion is written first.
- The negative ion is written next and a suffix is added at the end of the negative ion. The suffix written is '-ide'.
- In case of polyatomic ions, the name of the ion is written as such. For Example:
 is named as hydroxide,
is named as hydroxide,
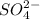 is named as sulfate and so on..
is named as sulfate and so on..
Hence, the name of
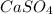 is calcium sulfate.
is calcium sulfate.