Answer:
(a)
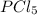
Step-by-step explanation:
Hello,
In this case, such nomenclature expresses the amount of both phosphorous and chlorine atoms via prefixes for the sub indexes of each atom into the molecule, thus, as phosphorous is prefixless one infers that there is just one phosphorous and five chlorides since the prefix for such atom is penta. Therefore, the correct formula is:
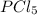
Hence, the answer is (a)
Best regards.