Answer: The correct answer will be 5 moles, because according to the stoichiometric ratio, 5 moles of oxygen produce 6 moles of water.
Step-by-step explanation:
The balanced equation is:
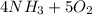 ⇒
⇒
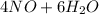
As you can see in the balanced reaction, it is necessary 5 moles of oxygen for obtain 6 moles of water. This stoichiometric ratio can be used for calculate any amount of produced water, once you have a specific amount of oxygen.