Answer :
(a) The density of mercury is, 13.6 g/ml
(b) The mass of 120.0 ml of mercury is, 1632 grams
Explanation :
(a) Now we have to calculate the density of mercury.
Given :
Volume of mercury = 25.0 ml
Mass of mercury = 340.0 g
Formula used :
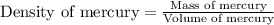
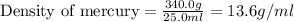
Therefore, the density of mercury is, 13.6 g/ml
(b) Now we have to calculate the mass of 120.0 ml of mercury.
As, 25.0 ml of mercury has mass = 340.0 g
So, 120.0 ml of mercury has mass =
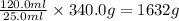
Therefore, the mass of 120.0 ml of mercury is, 1632 grams