Answer: The mass of oxygen is 40300 g, hydrogen is 11160 g, nitrogen is 6200 g, calcium is 1860 g, phosphorus is 992 g and other elements is 744 g in the body of 62 kg person.
Step-by-step explanation:
We are given:
Mass of body = 62 kg = 62000 g (Conversion factor: 1 kg = 1000 g)
Mass percent of oxygen = 65 %
This means that 65 grams of oxygen is present in 100 grams of human body.
By applying unitary method, we get:
In 100 g of human body, the mass of oxygen present is 65 grams.
So, in 62000 grams of human body, the mass of oxygen present will be =
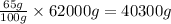
Mass of oxygen present = 40300 g
Mass percent of hydrogen = 18 %
This means that 18 grams of hydrogen is present in 100 grams of human body.
By applying unitary method, we get:
In 100 g of human body, the mass of hydrogen present is 18 grams.
So, in 62000 grams of human body, the mass of hydrogen present will be =
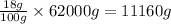
Mass of hydrogen present = 11160 g
Mass percent of nitrogen = 10 %
This means that 10 grams of nitrogen is present in 100 grams of human body.
By applying unitary method, we get:
In 100 g of human body, the mass of nitrogen present is 10 grams.
So, in 62000 grams of human body, the mass of nitrogen present will be =
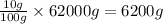
Mass of nitrogen present = 6200 g
Mass percent of calcium = 3 %
This means that 3 grams of calcium is present in 100 grams of human body.
By applying unitary method, we get:
In 100 g of human body, the mass of calcium present is 3 grams.
So, in 62000 grams of human body, the mass of calcium present will be =
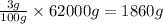
Mass of calcium present = 1860 g
Mass percent of phosphorus = 1.6 %
This means that 1.6 grams of phosphorus is present in 100 grams of human body.
By applying unitary method, we get:
In 100 g of human body, the mass of phosphorus present is 1.6 grams.
So, in 62000 grams of human body, the mass of phosphorus present will be =
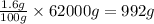
Mass of phosphorus present = 992 g
Mass percent of other elements = 1.2 %
This means that 1.2 grams of other elements is present in 100 grams of human body.
By applying unitary method, we get:
In 100 g of human body, the mass of other elements present is 1.2 grams.
So, in 62000 grams of human body, the mass of other elements present will be =
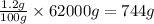
Mass of other elements present = 744 g
Hence, the mass of oxygen is 40300 g, hydrogen is 11160 g, nitrogen is 6200 g, calcium is 1860 g, phosphorus is 992 g and other elements is 744 g in the body of 62 kg person.