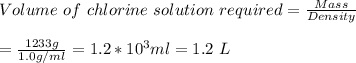Answer:
Volume of chlorine solution required is around 1.2 L
Step-by-step explanation:
Volume of water in the pool = 2.0*10^4 gal
1 gal = 3.79 L
Therefore, volume of water in the pool in units of Liters would be:
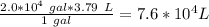
Density of water = 1 g/ml = 1000 g/L
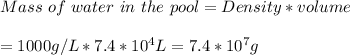
The accepted concentration of chlorine = 1 g/ 10⁶ g water
Therefore amount of chlorine required to disinfect the pool water would be:
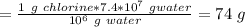
The given solution is 6.0% w/w chlorine i.e.
6.0 g chlorine in 100 g solution
Therefore, amount of solution corresponding to 74 g chlorine would be:
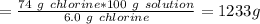
Density of the solution = 1 g/ml