Answer: The mass of osmium in kilograms and in pounds are 318.9141 kg and 703.085 pounds.
Step-by-step explanation:
To calculate the volume of sphere, we use the formula:
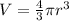
where,
r = radius of sphere
We are given:
Radius of osmium = 15 cm
Volume of osmium =
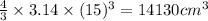
Density of osmium =
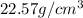
To calculate mass of a substance, we use the equation:

Putting values in above equation, we get:
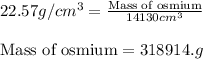
To convert the given mass into kilo grams and pounds, we use the conversion factors:
1 kg = 1000 grams
So,
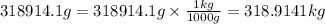
And,
1 pound = 453.592 g
So,
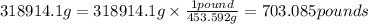
Hence, the mass of osmium in kilograms and in pounds are 318.9141 kg and 703.085 pounds.