Answer:
84.43 milliliters of 3.75 M Sulfuric acid are needed.
Step-by-step explanation:
Moles of barium oxide =
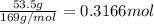
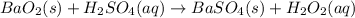
According to reaction, 1 mole of barium oxide reacts with 1 mole of sulfuric acid.
Then 0.3166 moles of barium oxide will react with:
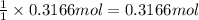 of sulfuric acid.
of sulfuric acid.
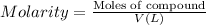
Where: V = Volume of the solution in Liters
Moles of sulfuric acid = 0.3166 mol
Volume of the sulfuric acid solution = V = ?
Molarity of sulfuric acid = 3.75 M
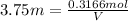
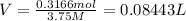
0.08443 L = 84.43 mL (1 L = 1000 mL)
84.43 milliliters of 3.75 M Sulfuric acid are needed.