Step-by-step explanation:
Atomic number of hydrogen is 1 as it is the very first element of periodic table.
Hence, electronic configuration of hydrogen is
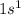 .
.
On the other hand, second element of the periodic table is helium and its atomic number is 2. Helium is also known as a noble gas.
Therefore, electronic configuration of helium is
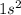 .
.
Thus, we can conclude that electronic configuration of hydrogen and helium is
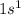 and
and
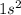 .
.