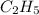Step-by-step explanation:
Empirical formula is defined as the formula in which atoms in a compound are present in simplest whole number ratios.
For the given options:
Acetic acid having molecular formula
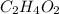 . To write the empirical formula, we divide each subscript by 2.
. To write the empirical formula, we divide each subscript by 2.
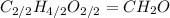
Hence, the empirical formula for acetic acid is
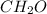
Citric acid having molecular formula
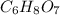 . To write the empirical formula, we divide each subscript by 1.
. To write the empirical formula, we divide each subscript by 1.
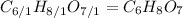
Hence, the empirical formula for citric acid is
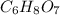
Hydrazine having molecular formula
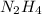 . To write the empirical formula, we divide each subscript by 2.
. To write the empirical formula, we divide each subscript by 2.
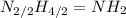
Hence, the empirical formula for hydrazine is
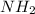
Nicotine having molecular formula
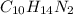 . To write the empirical formula, we divide each subscript by 2.
. To write the empirical formula, we divide each subscript by 2.
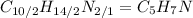
Hence, the empirical formula for nicotine is
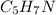
Butane having molecular formula
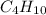 . To write the empirical formula, we divide each subscript by 2.
. To write the empirical formula, we divide each subscript by 2.
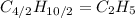
Hence, the empirical formula for butane is