Answer: The equations are written below.
Step-by-step explanation:
Single displacement reaction is defined as the reaction in which more reactive element displaces a less reactive element from its chemical reaction.
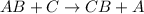
Element C is more reactive than element A.
Double displacement reaction is defined as the reaction in which exchange of ions takes place.
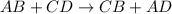
Decomposition reaction is defined as the reaction in which a large substance breaks down into smaller substances.
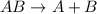
Synthesis reaction is defined as the reaction in which smaller substances combine in their elemental state to form a larger substance.
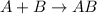
For the given options:
- Option 1: Iron (III) bromide reacting with ammonium sulfide to produce iron (III) sulfide and ammonium bromide
The balanced chemical equation follows:
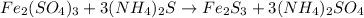
This is considered as double displacement reaction.
- Option 2: Calcium oxide reacting with diphosphorus pentoxide to produce calcium phosphate
The balanced chemical equation follows:
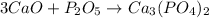
This is considered as synthesis reaction.
- Option 3: Magnesium chloride reacting with silver nitrate to produce magnesium nitrate and silver chloride
The balanced chemical equation follows:
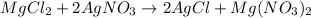
This is considered as double displacement reaction.
- Option 4: Sodium carbonate reacting with sulfuric acid to produce sodium sulfate, carbon dioxide and water
The balanced chemical equation follows:
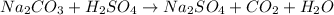
This is considered as double displacement reaction.
- Option 5: Iron(II) sulfide reacting with hydrochloric acid to produce iron (II) chloride and hydrogen sulfide
The balanced chemical equation follows:
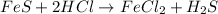
This is considered as double displacement reaction.
Hence, the reactions are written above.