Answer: This violates the law of constant composition.
Step-by-step explanation:
Dalton's theory is based on mainly two laws, which are law of conservation of mass and law of constant composition.
Law of constant composition states that a compound always contain the elements in the fixed ratio by their masses.
For Example: In water
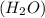 , the hydrogen and oxygen are present in the fixed ratio of 1 : 9 by their mass.
, the hydrogen and oxygen are present in the fixed ratio of 1 : 9 by their mass.
We are given:
A sample of titanium dioxide having 59.95 % of titanium and another sample of titanium dioxide having 60.10 % of titanium.
As, the compound is titanium dioxide. So, the mass percent of titanium must remain the same in both the elements.
Hence, this violates the law of constant composition.