Answer:
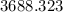 years
years
Step-by-step explanation:
Given-
Half life of
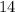 C
C
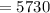 years
years
As we know -
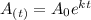
Where
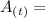 Mass of radioactive carbon after a time period "t"
Mass of radioactive carbon after a time period "t"
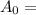 initial mass of radioactive carbon
initial mass of radioactive carbon
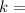 radioactive decay constant
radioactive decay constant
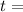 time
time
First we will find the value of "k"
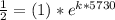
On solving, we get -
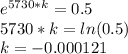
Now, when mass of 14C becomes
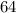 % of the plant material on earth today, then its age would be
% of the plant material on earth today, then its age would be
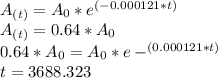 years
years