Answer:
Combustion energy for one mole of ethylene = 1410.51 kJ/mol
Step-by-step explanation:
Given data
heat capacity of bomb calorimeter = 2.47kJ/K
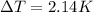
molecular weight of
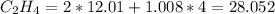
Total energy released after burning 0.105 g sample is = 2.47 *2.14 =5.28 kJ
Combustion energy for one mole of ethylene
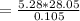
= 1410.51 kJ/mol