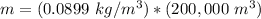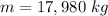Answer:
The mass of the hydrogen is 17,980 kilograms
Step-by-step explanation:
Remember that the density of a material is a function of its mass and its volume. This means that:
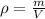
 is the density
is the density
m is the mass of the object
V is the volume of the object
In this case we have that:
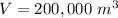
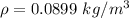
Then:
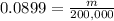
Now we solve the equation for m