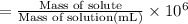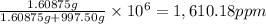Answer:
Mole fraction of salt is 0.00049.
The concentration of the salt solution in percent by mass is 0.16%.
The concentration of the salt solution in parts per million is 1,610.18 .
Step-by-step explanation:
Molarity of the NaCl solution =
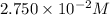
Moles of NaCl =
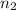
Volume of the solution = 1.000 L
Molarity=
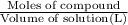
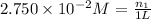
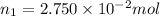
Mass of
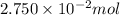 of NaCl :
of NaCl :
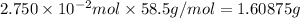
Mass of water = m
Density of water = 0.9982 g/mL
Volume of water = 999.3 mL
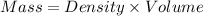
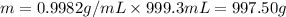
Moles of water =
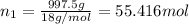
Mole fraction of salt =
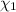
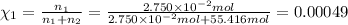
Percentage by mass:
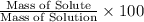
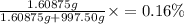
The concentration of the salt solution in percent by mass is 0.16%.
The concentration of the salt solution in parts per million.