1a. The solubility of calcium chloride (CaCl2) at 5°C is 60 g/100 g H2O.
We can see that the image shows a graph with several curves representing the solubilities of different salts in water at various temperatures.
We take a look at 5°C and look for the horizontal axis labeled "Temperature (°C)" and find the point corresponding to 5°C. We trace the curve from the 5°C point on the horizontal axis, follow the curve labeled
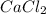 upwards until it intersects the vertical axis labeled "Solubility (g of salt in 100 g
upwards until it intersects the vertical axis labeled "Solubility (g of salt in 100 g
 .
.
The point of intersection between the
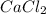 curve and the vertical axis indicates the solubility at 5°C and in our case around 60 g/100 g
curve and the vertical axis indicates the solubility at 5°C and in our case around 60 g/100 g
 .
.
In conclusion, with a confidence level based on the image resolution, we can say that the solubility of calcium chloride at 5°C is approximately 60 g per 100 g of water.