Step-by-step explanation:
It is known that an electron pair represents a domain. Hybridization of
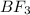 will be calculated as follows.
will be calculated as follows.
Hybridization =
![(1)/(2)[\text{Valence electrons of central atom + no. of bonded atoms + negative charge - positive charge}]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/hwog6vr0tiyzkcthsbjjpfv1506z553z1b.png)
Valence electrons of boron are 3 and no. of bonded atoms in a
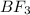 molecule is 3.
molecule is 3.
Therefore, calculate hybridization of
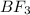 as follows.
as follows.
Hybridization =
![(1)/(2)[\text{Valence electrons of central atom + no. of bonded atoms + negative charge - positive charge}]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/hwog6vr0tiyzkcthsbjjpfv1506z553z1b.png)
=
![(1)/(2)[3 + 3 + 0]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/r88d679w0yj27k4hy0gwtrvadjg8mv7819.png)
= 3
Hence, hybridization of
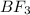 is
is
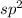 , that is, trigonal planar.
, that is, trigonal planar.
Thus, we can conclude that the given image represents that it is a trigonal planar molecule with three domains.