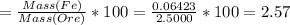Answer:
% Iron in the ore = 2.57%
Step-by-step explanation:
The redox reaction involving the oxidation of Fe2+ to Fe3+ in the presence of Na2Cr2O7 is:
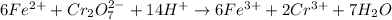
Based on the reaction stoichiometry:
6 moles of Fe2+ requires with 1 mole of Cr2O7^2-
Moles of Cr2O7^2- required in the titration:
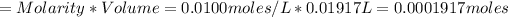
Therefore, moles of Fe2+ present is:
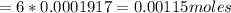
Atomic mass of Fe = 55.85 g/mol
Mass of Fe present in the ore is:
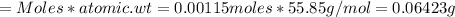
%Fe in the ore is: