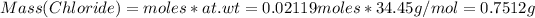Answer:
Mass% chloride ion = 29.32%
Step-by-step explanation:
Given:
Mass of alkali metal chloride sample = 2.5624 g
Mass of the precipitate = 3.03707 g
To determine:
The mass percent of chloride ion in the sample
Calculation:
The reaction of alkali metal with silver nitrate results in the precipitation of the chloride ion as silver chloride (AgCl).
Molecular weight of AgCl =143.32 g/mol
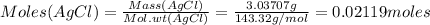
Since 1 mole of AgCl contains 1 mole of Cl, therefore:
# moles of Cl = 0.02119 moles
At wt of Cl = 35.45 g/mol