Answer:
Lewis structure for isomers of butane has been given below.
Step-by-step explanation:
Butane is a saturated alkane with molecular formula
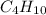 . Due to different positing of methyl groups, positional isomerism exists in butane.
. Due to different positing of methyl groups, positional isomerism exists in butane.
Butane has two positional isomers with same molecular formula. One is n-butane and another one is isobutane. Lewis structures of these two isomers have been given below.