Answer: The actual yield of hydrogen gas in the following reaction is 4.974 grams.
Step-by-step explanation:
The chemical equation for the reaction of sodium and hydrofluoric acid follows:
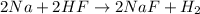
As, hydrofluoric acid is present in excess. So, it is considered as an excess reagent and sodium is considered as a limiting reagent because it limits the formation of products.
By Stoichiometry of the reaction:
2 moles of sodium is producing 1 mole of hydrogen gas.
So, 5.75 moles of sodium will produce =
 of hydrogen gas.
of hydrogen gas.
To calculate the actual yield of hydrogen gas, we use the equation:
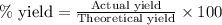
% yield of reaction = 86.5 %
Theoretical yield of hydrogen gas = 2.875 moles
Putting values in above equation, we get:
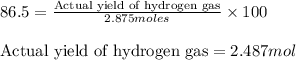
Now, to calculate the mass of hydrogen gas, we use the equation:

Moles of hydrogen gas = 2.487 mol
Molar mass of hydrogen gas = 2g
Putting values in above equation, we get:
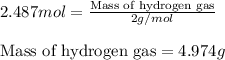
Hence, the actual yield of hydrogen gas in the following reaction is 4.974 grams.