Answer:
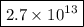
Step-by-step explanation:
Probability of one ²H is 0.00015 = 1.5× 10⁻⁴
Probability of two ²H is 1.5× 10⁻⁴ × 1.5 × 10⁻⁴ = 2.25 × 10⁻⁸
Probability of two ²H plus one ¹⁸O is 2.25 × 10⁻⁸ × 0.0020
= 4.5× 10⁻¹¹
Molecules in 1.0 mol water = 1.0 × 6.022 × 10²³ × 4.5 × 10¹¹
= 2.7 × 10¹³
There are 2.7 × 10¹³ molecules of ²H₂¹⁸O in 1.0 mol of water.