Answer:
12% is the mass percent of methanol in the solution.
Step-by-step explanation:
Mass of water = 100.0 g
Mass of NaCl = 10.0 g
Mass of methanol = 15.0 g
Mass of the solution = 100.0 g+ 10.0 g+ 15.0 g = 125.0 g
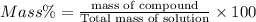
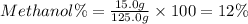
12% is the mass percent of methanol in the solution.