Step-by-step explanation:
As it is known that relation between specific heat and heat energy is as follows.
Q =
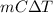
where, Q = heat energy
m = mass
C = specific heat capacity = 4.18
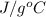
 = change in temperature
= change in temperature
Therefore, putting the given values into the above formula as follows.
Q =
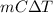
=
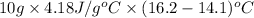
= 87.78 J
Thus, we can conclude that the heat energy needed to raise the temperature of 10.00 g of liquid water from
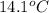 to
to
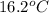 is 87.78 J.
is 87.78 J.