Answer:
Red light
Step-by-step explanation:
The energy emitted during an electron transition in an atom of hydrogen is given by
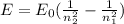
where
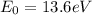 is the energy of the lowest level
is the energy of the lowest level
n1 and n2 are the numbers corresponding to the two levels
Here we have
n1 = 3
n2 = 2
So the energy of the emitted photon is
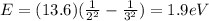
Converting into Joules,
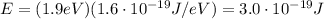
And now we can find the wavelength of the emitted photon by using the equation
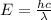
where h is the Planck constant and c is the speed of light. Solving for
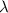 ,
,
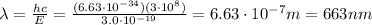
And this wavelength corresponds to red light.