Answer:
D. positive/accept
Step-by-step explanation:
a) The standard gibbs free energy (ΔG⁰) is related to the standard cell potential (E⁰) as follows:
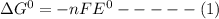
where n = number of electrons
F = faraday constant
The given half reaction reaction has ΔG⁰ = -81 kJ/mol. Based on equation (1) a negative value for the free energy can be obtained only if E⁰ is positive.
Thus, the negative value of ΔG°' results from the very positive value of E⁰
b) The value of E⁰ is the standard reduction potential which reflects the tendency of the system to accept electrons and get reduced. Higher (more positive) the value, greater will be the tendency to accept electrons.
Therefore, the positive E⁰ value of the redox couple NO3–/NO2– reflects the strong tendency of nitrate to accept electrons.