Answer: Thus the value of the equilibrium constant is

Step-by-step explanation:
Equilibrium constant is the ratio of the concentration of products to the concentration of reactants each term raised to its stochiometric coefficients.
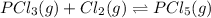
At eqm. conc. (0.20) M (0.25) M (1.20) M
The expression for equilibrium constant for this reaction will be,
![K_c=([PCl_5])/([PCl_3][Cl_2])](https://img.qammunity.org/2020/formulas/chemistry/college/8l4jdg6ls22il3qpcc72id4d94uchcinhp.png)
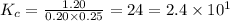
The figures in a number which express the value -the magnitude of a quantity to a specific degree of accuracy is known as significant digits.
Thus the value of the equilibrium constant is
