Answer:
5.112 grams of simple sugar glucose is produced by the reaction of 7.50 g of carbon dioxide.
Step-by-step explanation:
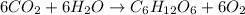
Moles of carbon dioxide =
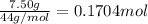
According to reaction 6 moles of carbon dioxide gives 1 mole of glucose.
Then 0.1704 moles of carbon dioxide will give:
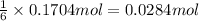 of glucose
of glucose
Mass of 0.0284 moles of glucose:
0.0284 mol 180 g/mol =5.112 g[/tex]
5.112 grams of simple sugar glucose is produced by the reaction of 7.50 g of carbon dioxide.