Answer:
9419.3 years.
Explanation:
Let the initial amount of C-14 be 100 units.
We have been given that a hiker in Africa discovers a skull that contains 32% of its original amount of C-14. We are asked to find the age of the skull.
We will use half life formula to solve our given problem.
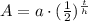 , where,
, where,
A = Amount left after t years,
a = Initial amount,
t = time,
h = Half life.
We know that half-life of C-14 is 5730 years.
32% of 100 units would be 32.
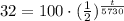
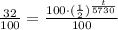
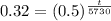
Now, we will take natural log of both sides.
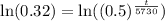
Using log property
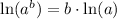 , we will get:
, we will get:
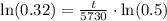
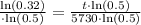
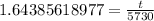
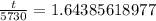
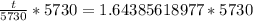
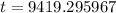
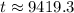
Therefore, the age of skull is approximately 9419.3 years.