Answer:
First, precipitate of AgCl is formed. Second, a soluble complex of silver and ammonia is formed. Third, AgCl is reproduced due to disappearance of ammonia complex in presence of
 .
.
Step-by-step explanation:
In presence of NaCl,
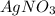 forms an insoluble precipitate of AgCl.
forms an insoluble precipitate of AgCl.
Reaction:
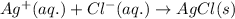
In presence of
 , AgCl gets dissolved into solution due to formation of soluble
, AgCl gets dissolved into solution due to formation of soluble
![[Ag(NH_(3))_(2)]^(+)](https://img.qammunity.org/2020/formulas/chemistry/college/oegkv0n2sf03pjj088xhstr9zgkw2m2sv5.png) complex.
complex.
Reaction:
![AgCl(s)+2NH_(3)(aq.)\rightarrow [Ag(NH_(3))_(2)]^(+)(aq.)+ Cl^(-)(aq.)](https://img.qammunity.org/2020/formulas/chemistry/college/7hntui1sqheyklbsqjszufog3nvijd7giv.png)
In presence of
 ,
,
![[Ag(NH_(3))_(2)]^(+)](https://img.qammunity.org/2020/formulas/chemistry/college/oegkv0n2sf03pjj088xhstr9zgkw2m2sv5.png) complex gets destroyed and free
complex gets destroyed and free
 again reacts with free
again reacts with free
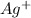 to produce insoluble AgCl
to produce insoluble AgCl
Reaction:
![[Ag(NH_(3))_(2)]^(+)(aq.)+2H^(+)(aq.)+Cl^(-)(aq.)\rightarrow AgCl(s)+2NH_(4)^(+)(aq.)](https://img.qammunity.org/2020/formulas/chemistry/college/qmi1ep3m28epprcj9qm0pkaop00tlde4zt.png)