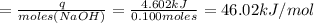Answer:
Heat of the reaction per mole of NaOH = 46.02 kJ/mol
Step-by-step explanation:
The reaction between HCl (strong acid) and NaOH(strong base) is a neutralization reaction which yields a salt NaCl and water
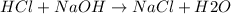
The heat (q) of a reaction is given as:
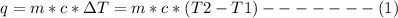
where m = mass of the system
c = specific heat
T1 and T2 are the initial and final temperatures
It is given that:
Volume of HCl = 500.0 ml
Volume of NaOH = 500.0 ml
Density of HCl and NaOH = 1.000 g/ml
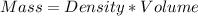
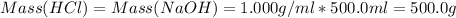
Total mass of the solutions, m = 500.0 +500.0 = 1000.0 g
c = 4.184 J/g/c
T1 = 25.6 C
T2 = 26.70 C
Substituting appropriate values in equation (1) gives:
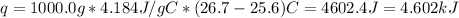
Now, the number of moles of NaOH is:
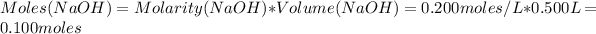
Heat of reaction/mole NaOH is: