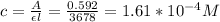Answer:
FAS concentration = 1.61*10^-4M
Step-by-step explanation:
Beer Lambert's law relates the absorbance (A) of a substance to its concentration (c) as:

where ε = molar absorption coefficient
l = path length
A plot of 'A' vs 'c' gives a straight line with slope = εl
In addition absorbance (A) is related to % Transmittance (%T) as:
A = 2-log%T----(2)
For the FAS solution, the corresponding calibration fit is given as:
y = 3678(x) + 0.056
This implies that the slope = εl = 3678
It is given that %T = 25.6%
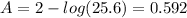
Based on equation(1):