Answer:
ok:
Step-by-step explanation:
We have:
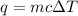
we know that q = -5000 J (it is negative since you release energy)
m = 320 g
and c = 376 J/kg.
We need to be careful before plugging them in. Since the units are J/kg for c, but we are talking about grams for m, we need to convert. I will just divide c by 1000 to get c = 0.376 J/g
We can now plug in:
(-5000 J) = (320 g) * (0.376 J/g) *

We can solve that
 = -41.5555
= -41.5555
So the final temperature is
 which equals around 8.4°C.
which equals around 8.4°C.