Step-by-step explanation:
Equation for half-life of a first order reaction is as follows.
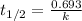
where, k = rate constant
Hence, it shows that there is no relation between temperature and half-life of a reaction.
Also, in second or third order reaction there is no relation between temperature and half life. As half-life is independent of temperature.
Thus, any increase or decrease in temperature is not going to affect the half-life time of a chemical.