Answer:
C) 900 J
Step-by-step explanation:
Calculate the heat needed to heat 0.5 kg of aluminum.
As the problem does not tell us that there is a change of state during this process the formula of heat that we must use is
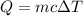
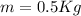
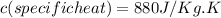 (this value is a constant for aluminum)
(this value is a constant for aluminum)
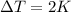
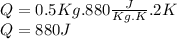
The exact amount of heat to heat 0.5 kg of heat is 880 J, but this value could be rounded to the nearest hundred that would be 900 J